Validation and Qualification Services
Working closely with our Regulatory Practice, RCM’s Validation and Qualification (V&Q) Practice can bring the latest methods and approaches to meet the everchanging regulations for validation.
RCM offers a complete range of services from Computer Systems and Process Validation to Facility and Equipment Qualification. We can assist you with evaluating where and how the latest approaches – Risk Based, Computer Software Assurance (CSA), or Continuous Validation – could be leveraged in your organization.
Whether this is your first validation project, or you have a mature process, RCM can evaluate how best to optimize your process, and save you time,

The table above lists required or related regulations and guidance. These are areas where RCM Life Sciences has subject matter experts (SME) with specific knowledge to assist in Validation and Qualification Services.
Computer System Validation (CSV)
RCM CSV solutions promote efficiencies, risk based and least burdensome approaches while always ensuring requirements of validation (including Part 11 / Annex 11) are met with a high degree of data integrity.
We know the difference between Agile and Traditional validation methods, such a V, Inverted V and Hybrid. Our Validation and Qualification Practice consultant are experienced working with FDA and meeting Regulations. Interpreting the regulations is necessary to ensure that you build your product following you intended use. We can assist you with new strategies, techniques, and methods to provide assurance that your system will be compliant and meet all data and system regulations.
Try us and see what services our Validation and Qualification Practice has to offer.
Support or Link
What does the FDA’s Guidance on CSA mean to you? Vodori Case Study
Whitepaper CSA Kathleen Warner
RCM’s Validation and Qualification (V&Q) Practice offers the following services: Computer System Validation (CSV) and Computer Software Assurance (CSA).
We leverage traditional methodologies, as well as new methodologies, when performing any type of validation.
Whether it be:
- Agile method (i.e., integrate test, integrate test, integrate test…),
- Continuous V Model .
- Hybrid
- Waterfall
Or other new methodologies, such as:
- Automated testing
- Continuous Validation
- Cloud computing
We are the experts in all areas of Validation and Qualification Services.
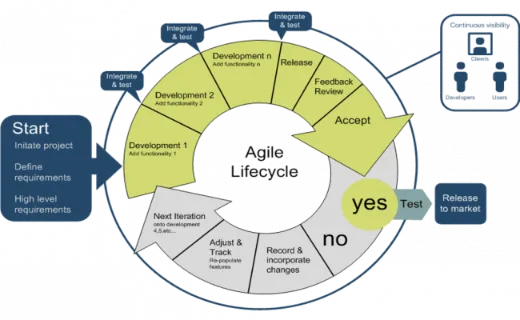
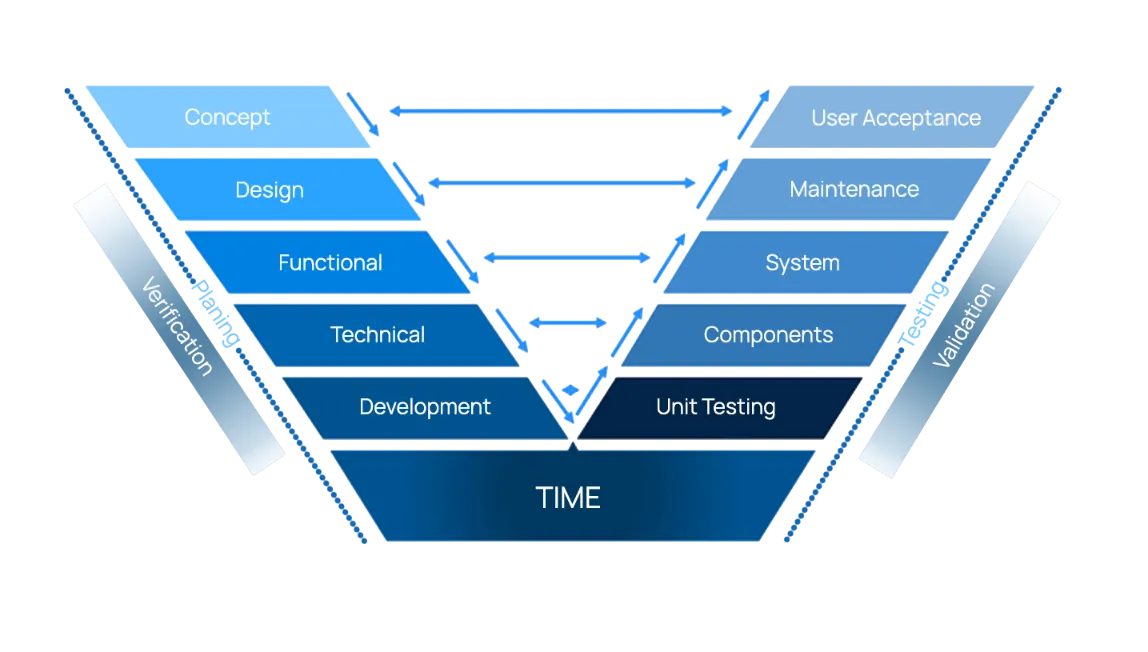
Deciding which validation method fits your environment can be confusing and worrisome.
Simply put, the Agile method uses an innovative, less structured, more flexible, iterative process for software development. The team oversees and manages the software development activities.
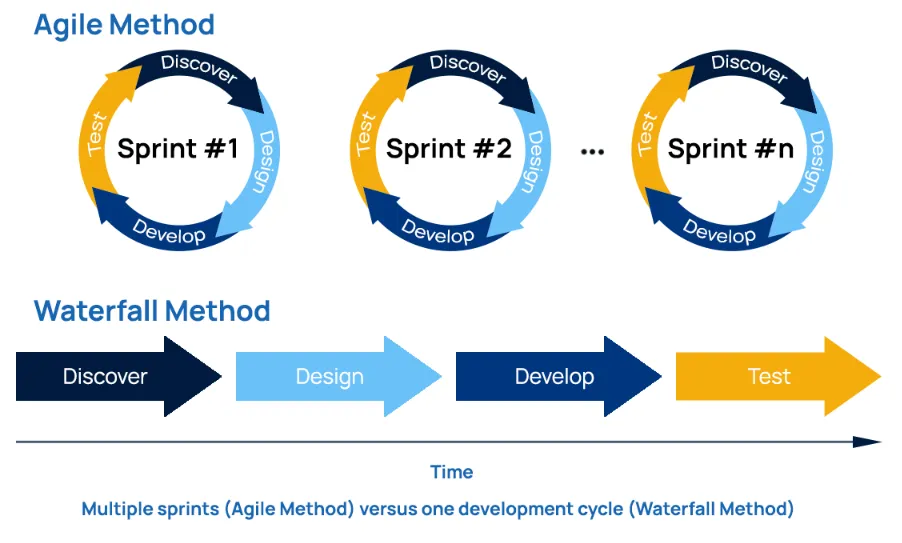
Waterfall method is a traditional, structured, linear approach for software development . Business analysts and project manages are important to develop processes, create requirements, and manage the project deliverables and timeline.
Process Validation
RCM’s full range of Process Validation capabilities include Process Validation, Qualification and Continuous Process Verification. It all starts with an assessment to understand your organization and identify gaps that are related to process quality and controls, data integrity and business process management.

RCM can support you by authoring Protocols, such as Process Performance Qualification (PPQ), developing a Master Validation Plan, and managing your entire Process Validation project – from risk assessment to requirements gathering straight through to implementation and continuous process verification.
Clean Room Validation
RCM has experience and knowledge of how to be compliant and also what you need to know to be certified.
We start with questions like are you certified and/or do you plan on becoming certified?
Are you trained on the regulatory requirements including Annex1, 14644-1, 21CFR11, 21501-4, USP 788?
Do you understand the validation lifecycle of a clean room? If you have questions on Cleanrooms, certification, validation we have answers!

Facilities Qualification
Including but not limited to: HVAC, Purified RO/RI Water, Water for Injection (WFI), SIP/CIP, Utility services, clean room, etc.
Equipment Qualification both Lab and Manufacturing (with or w/o integrated software)
Equipment Qualification
Including but not limited to: Autoclave, Biosafety Cabinet, Blenders and Mixers,
Capping Machine, Capsule Filling Machine, Checkweigher, Cleaned-In Place Tanks, Depyrogenation Oven, Depyrogenation Tunnel, Fermentor (Bioreactor), Filling Machine (Ampule/Vials), Fume Hood, Liquid Nitrogen Freezers, Lyophilizer, Mills and Micronizers, Spray Granulator, Tablet Coater, Tablet Presses

Equipment qualification process:
- Design qualification
- Installation qualification
- Operation qualification
- Performance qualification
Advisory services on the regulatory requirements including Annex1, 14644-1, 21CFR11, 21501-4, USP 788
RCM has resources with expertise to help you develop your Cleaning Validation Strategy kit, that includes:
- Cleaning Validation Gap Analysis
- Design of Experiments (DOE)
- Protocol generation, execution and reporting services.
- Equipment Matrix / Family / Grouping Strategy
- Cleaning Cycle Development
- Contamination Evaluation / Response /Remediation / Controls
- Cleaning Validation program audits
